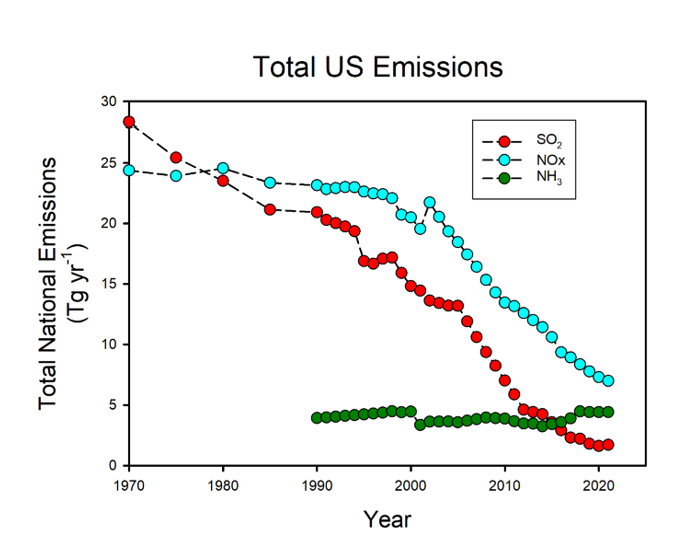
Acid deposition and its effects on ecosystems is a long-standing research interest. Acid deposition is elevated concentrations of strong acids deposited to the Earth’s surface as a result to emissions of fossil fuels and agricultural activities. Acid deposition has acidified soil and water in sensitive regions resulting in adverse effects on soils, trees and aquatic organisms. Emissions of acidifying air pollutants peaked in the U.S. in the 1970s for sulfur dioxide and in the early 2000s for nitrogen oxides and have decreased markedly in recent decades, due to air quality legislation and rules (Figure 1). I recently published a synthesis of acid and mercury deposition and its effects for the Encyclopedia of Biodiversity (Driscoll 2022). As part of my ongoing work, I am examining the recovery of ecosystems in the eastern U.S. from historical acid deposition. With colleagues (Jana Milford, Daven Heinze, University of Colorado), I am also working on a critical review of ammonia emissions and its effects on atmospheric chemistry and deposition and ecosystems, and its management that will be published in 2024.
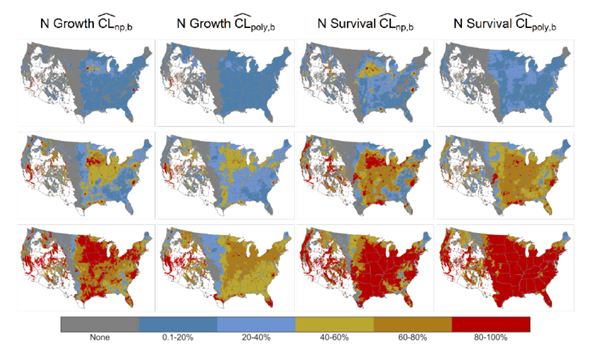
Critical loads are a determination of levels of atmospheric deposition, below which will alleviate adverse ecosystem effects for a specific period. I completed a critical loads project with funding from the Electric Power Research Institute. Working with Sonoma Technologies and partnering with a joint federal agency task force, including EPA, Research Triangle International, the National Park Service and the US Forest Service (US FS), we applied machine learning techniques to the USFS Forest Inventory Analysis to quantify critical loads of sulfur and nitrogen deposition for tree growth and survival for the coterminous US (Figure 2). This is an exciting project and may establish an approach that will be used to determine a secondary air quality standard for sulfur and nitrogen. This work is continuing through a new contract with EPRI to examine ozone effects on US forests. This project opens new opportunities for research on the application of machine learning techniques to characterize and quantify effects of air pollution on ecosystems.
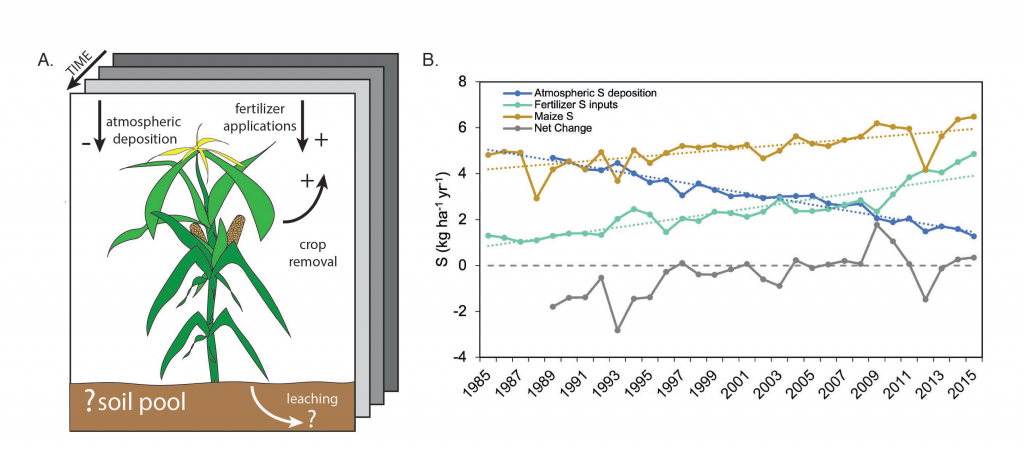
I have expanded my research on acid deposition to contrast this with effects of agricultural amendments of sulfur. Over the past few years, I have worked with Eve-Lyn Hinckley (University of Colorado) to characterize and quantify inputs and effects of agricultural application of sulfur on ecosystems, contrasting these with the well-established effects of atmospheric sulfur deposition (i.e., acid rain). We found in the Midwestern region of the U.S. fertilizer sulfur application rates for corn and soy have increased in recent decades to make up for decreases in atmospheric sulfur deposition (Hinckley and Driscoll 2022; Figure 3). The sulfur addition rate for agricultural applications is more intense at the local scale but the areal extent is smaller than for atmospheric sulfur deposition. This work will continue as we examine the roles of climate, hydrology, fertilizer application rates and other element cycles in modifying sulfur processes and flows within and down gradient of agricultural source areas.
I have expanded my research on acid deposition to contrast with effects of agricultural amendments of sulfur. Over the past few years, I have worked with Eve-Lyn Hinckley (University of Colorado) to characterize and quantify inputs and effects of agricultural application of sulfur on ecosystems, contrasting these with the well-established effects of atmospheric sulfur deposition (i.e., acid rain). We found in the Midwestern region of the U.S. fertilizer sulfur application rates for corn and soy have increased in recent decades to make up for decreases in atmospheric sulfur deposition (Hinckley and Driscoll in review). The sulfur addition rate for agricultural applications is more intense at the local scale but the areal extent is smaller than for atmospheric sulfur deposition. This work will continue as we examine the roles of climate, hydrology, fertilizer application rates and other element cycles in modifying sulfur processes and flows within and down gradient of agricultural source areas.
References:
*Driscoll, C. T. 2022. Acid and mercury deposition effects on forest and freshwater aquatic ecosystems. Pages 1-14 in S. A. Levin, editor. Encyclopedia of Biodiversity, third edition. Elsevier Inc., Waltham, MA: Academic Press. doi:10.1016/B978-0-12-384719-5.00303-8.
Hinckley, E. and C. T. Driscoll. 2022. Fertilizer applications replace atmospheric deposition to supply sulfur to Midwest croplands. Nature Communications Earth and Environment, 3(324). doi:10.1038/s43247-022-00662-9
Pavlovic, N. R., S.Y. Chang, J. Huang, K. Craig, C. Clark, K. Horn and C. T. Driscoll. 2022. Empirical nitrogen and sulfur critical loads of U.S. tree species and their uncertainties with machine learning. Science of the Total Environment, 857: 159252. doi:10.1016/j.scitotenv.2022.159252.
US Environmental Protection Agency. Air Quality System Data Mart [internet database] available via https://www.epa.gov/outdoor-air-quality-data. Accessed August 2023.